A Closer Look into Cell and Gene Therapies
Research and development in cell and gene therapies (CGTs) is growing at a rapid rate. Innovation in this sector brings a promise of life-changing therapies for complex or rare diseases such as cancer and genetic disorders. However, these advanced therapies also come with a high price tag, posing challenges to plan sponsors around drug therapy reimbursement. Plan sponsors will need to reimagine the way they approach drug therapy reimbursement and be innovatie in their management strategy.
What are Cell and Gene Therapies? Cellular therapy products involve extracting cells from a donor and altering these cells into a personalized therapy, which then can be re-injected into the patient.(1) Cell therapies may include cellular immunotherapies, cancer vaccines, and other types of both autologous and allogeneic cells. Human gene therapies involve modifying gene expression or altering the biological properties of living cells for therapeutic use.(2) In other words, gene therapies insert genes into the patient, usually through a viral factor, to “fix” a genetic deficit. These therapies can therefore have long-lasting effects than traditional medicines.
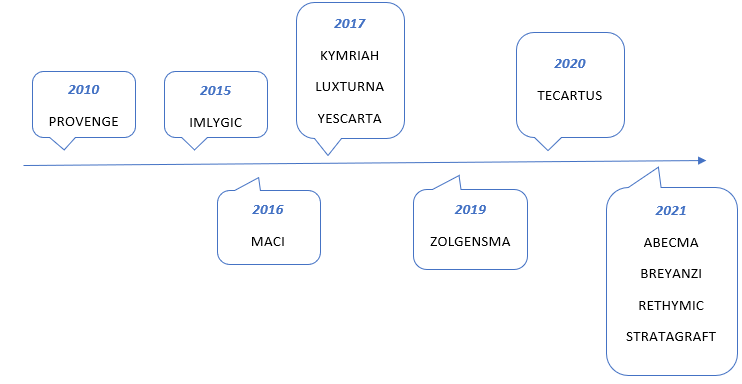
Approved FDA Products: There are currently 22 FDA Approved CGT products in the US.(3) Below is a snapshot of some therapies currently on the market now in chronological order.(3) See Figure 1 below.
Reimbursement for CGTs: Due to high costs, most coverage plans for CGTs involve value-based contracting, a model that allows plan sponsors to spread out payments over a longer period and receive discounts if a give therapy does not provide certain clinical outcomes and benefits. However, there are still challenges around innovative reimbursement models such as value-based contracting.
Contracts for gene therapies are typically for 2-3 years (4), but as the durability of treatments can extend beyond that, plans may be interested in extending the contract to a longer time frame. However, this is extremely difficult to determine.
The key concern is the fragmented US healthcare system that has multiple insurance plans and delivery systems, which does not allow for one payer to spread out the costs over a longer timeframe. As patients can switch insurance plans halfway through a contract, this creates challenges in cost burden for a payer to capture the full value, especially for smaller plans that may not have as many lives to offset the cost of a gene therapy.
With more than 3000 gene therapies (4) in development, plan sponsors need to continue the efforts to innovate reimbursement plans and contracts for better budget planning in the future.
Figure 1: Approved FDA Products